Red Light, Green Light: responses to cortisol levels in keto vs. longevity research
How a scientist interprets outcomes often depends on whether she thinks the outcome should be good or bad.
Cortisol levels make a good example.
In the context of low carb, ketogenic diets, the finding of slightly higher cortisol levels have been interpreted as a warning sign. In a recent post on our blog, I attempt to explain why the “red light” that Boston Children’s Hospital gave low carb diets is not justified.
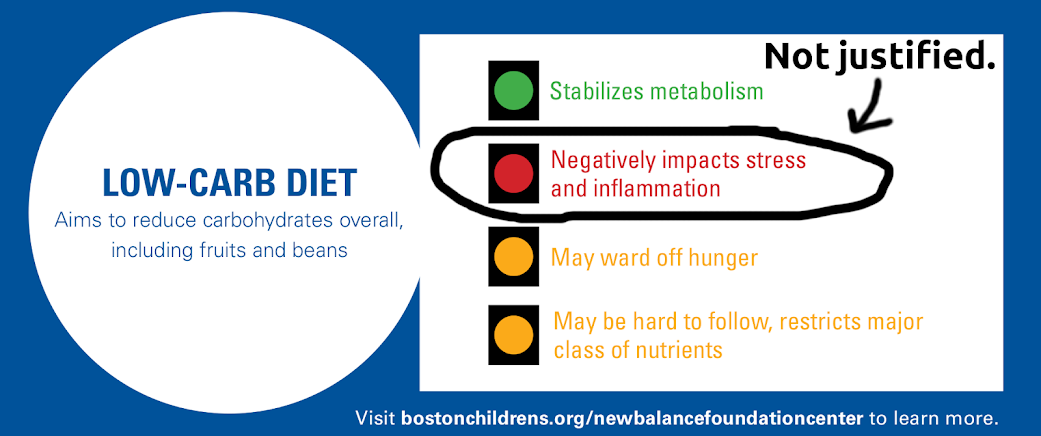
It is interesting to note that while cortisol sends up red flags for ketogenic diets, which mainstream medicine actively disapproves of, it sends up green flags in another context: longevity research.
Time and again I have come across glib statements in longevity papers saying that the beneficial, health-enhancing and lifespan-increasing effects of caloric restriction probably come in part from the moderately increased cortisol that is consistently seen in calorie restricted animals. The intuitiveness of the beneficial effects of cortisol is usually based on cortisol’s known anti-inflammatory action.
Here are just a few such quotes:
“The mechanisms responsible for calorie restriction–mediated beneficial effects on primary aging observed in rodents probably involve the metabolic adaptations to restriction itself, including… a modest increase in levels of circulating cortisol, which result in a reduction in systemic inflammation.” — Aging, adiposity, and calorie restriction. Fontana L, Klein S. JAMA. 2007 Mar 7;297(9):986-94.
“Even short-term DR can attenuate inflammation and affect metabolic and DNA repair pathways. Mechanisms by which DR suppresses peripheral inflammation include the elevation of glucocorticoids, lowering of glucose and activation of PPARs. Although the effects of DR are less understood in the brain, common pathways are emerging that link many normal aging inflammatory processes with age related diseases such as AD, cancer, diabetes and cardiovascular disease.” — Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. Morgan TE, Wong AM, Finch CE. Interdiscip Top Gerontol. 2007;35:83-97.
“Glucocorticoids are yet another class of hormones that may contribute to the anticarcinogenic action of DR [101, 102]. Total and/or free glucocorticoid levels are increased by DR [103–105]. Glucocorticoids suppress cellular proliferation and enhance apoptosis in a number of cell types, including osteoblasts, lymphocytes and keratinocytes (for reviews, see Weinstein [106], Herold et al. [107] and Budunova et al. [108]). In humans, glucocorticoids are effectively used for treating lymphoid neoplasms [109]. Importantly, adrenalectomy abolishes the protective effect of DR on skin and pulmonary carcinogenesis, while glucocorticoid replacement restores this protection [110–112]. — Can short-term dietary restriction and fasting have a long-term anticarcinogenic effect? Klebanov S. Interdiscip Top Gerontol. 2007;35:176-92.
Another mechanism by which CR may selectively exert it’s anti-inflammatory effects is via enhanced endogenous corticosteroid production (Sabatino et al. 1991). Chronic CR potentiates the diurnal elevation of plasma corticosterone. CR mice and rats have “moderately” but significantly higher daily mean plasma free corticosterone concentration than mice fed “ad libitum” throughout their lifespan…
It is well known that the hypothalamic–pituitary–adrenal axis and glucocorticoids in particular are essential in limiting and resolving the inflammatory process (Sapolsky et al. 2000). Glucocorticoids have pleiotropic inhibitory effects on the immune system and inflammatory gene expression (Rhen and Cidlowski 2005). In addition, treatment with pharmacological doses of exogenous glucocorticoids has been used to block many inflammatory and autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus, Graves’ disease, thyroiditis, glomerulonephritis, multiple sclerosis, and psoriasis.” — Neuroendocrine Factors in the Regulation of Inflammation: Excessive Adiposity and Calorie Restriction Luigi Fontana, Exp Gerontol. 2009; 44(1-2): 41–45.
I particularly like that last one, because of the use of the word “enhanced”, which connotes that the thing that has increased is surely a Good Thing.
Now of course, the longevity researchers can’t pick up on a significant difference between the animals that lived longer and those that didn’t and say: Well, the diet might be good for some things, but this difference warrants caution. They can’t do that because the end outcome is definitive. It’s true that there is a significant school of thought that says that longevity is a result of hormesis. The hormetic explanation amounts to “what doesn’t kill you makes you stronger.” That way a researcher gets to say that phenomenon X is bad for you, and that things that are bad for you improve your health. It is a way out of a paradox, as expressed nicely here:
“One well-known, exemplified response to stress is the hormonal increase in adrenal corticosterone levels in plasma during aging, where increases in these levels appear to be proportional to the degree of stress. Aged animals appear to have a diminished ability to attenuate the increase, causing the aged to have continually elevated plasma levels of corticosterones. These authors suggested that increased levels of corticosterone in aged rats result in hippocampal neuronal cell death, that is, the stage of exhaustion. However, this scenario in the glucocorticoid cascade hypothesis is obviously not applicable in the case of the CR paradigm, because CR results in an increased life span in spite of chronically elevated diurnal levels of serum corticosterone. This apparent contradiction makes the interrelation of glucocorticoid and aging far more complex than one might want to narrowly define it and needs other mechanistic explanations like stress resistance to resolve the disparity in responses.” — Stress resistance by caloric restriction for longevity. Yu BP, Chung HY. Ann N Y Acad Sci. 2001 Apr;928:39-47. (my emphasis)
As Carol Loffelmann recently said on twitter: Scientists who discover paradoxes should examine their initial assumptions.
So supposing we have a study showing that a group that looks healthier than the other groups in essentially every measure also has higher levels of cortisol. We can reason, as Ebbeling et al. do, that since higher cortisol is associated with bad health outcomes, the ketogenic diet may be dangerous, despite the other measures. Or we can reason, as the longevity researchers do (and as Zooko and I did), that since the group is healthier, higher cortisol must be exerting or reflecting a healthy process, and this may present a paradox that we as researchers have to resolve.
Allow me one further point:
The findings of higher cortisol in calorie restricted animals is itself a body of literature of relevance here. Anyone finding that their intervention moderately elevates cortisol can and should now say: Higher cortisol levels are found in animals whose lifespans have been increased experimentally by dietary intervention, and so this finding in our intervention could be indicative of a longevity-inducing effect.
